1 3 5 Hexatriene Pi Molecular Orbitals

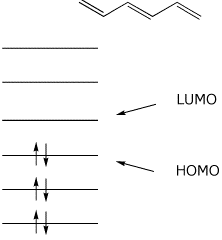
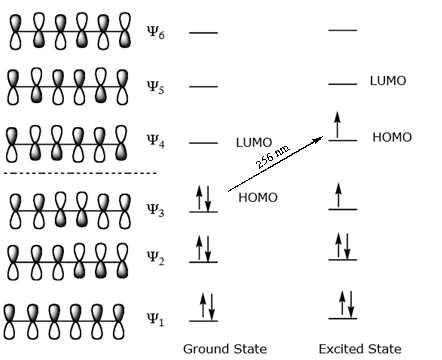
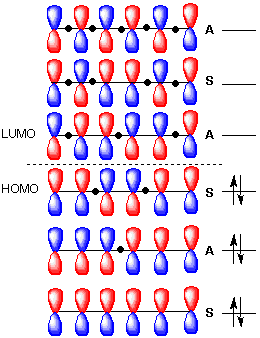
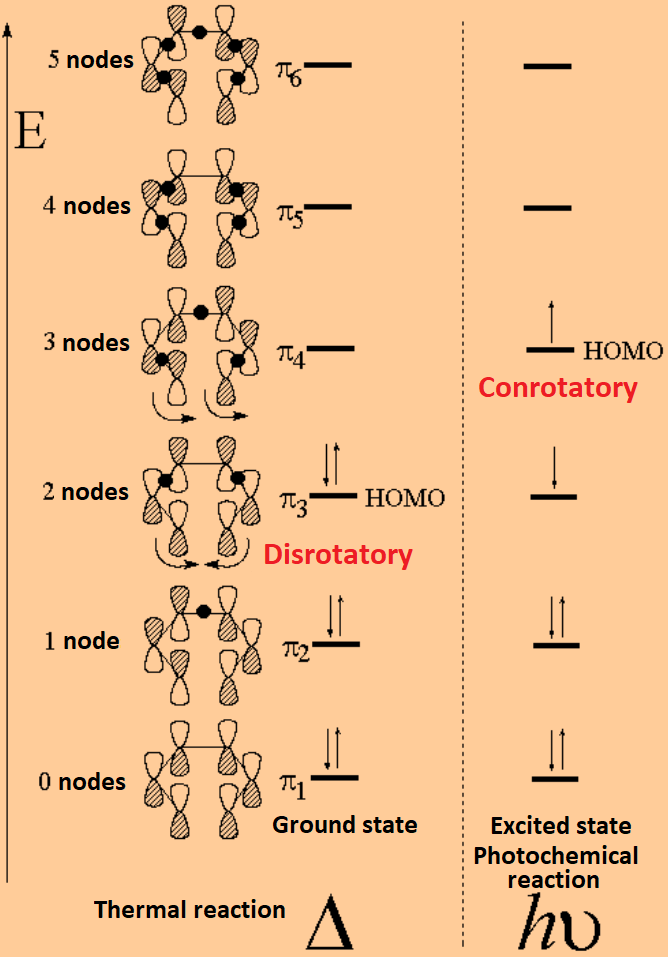
A write an energy level diagram indicating the number of pi molecular orbitals expected their relative energies the number of electrons expected in each orbital and identifying the homo and lumo.
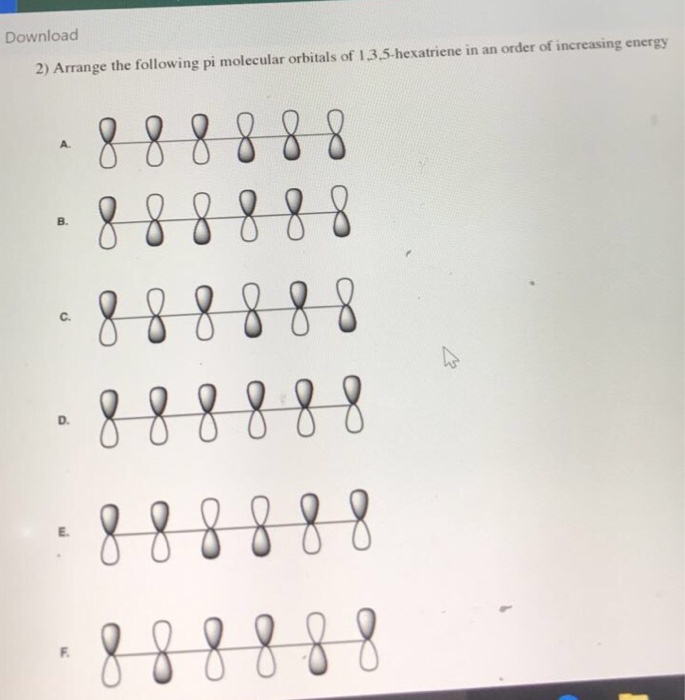
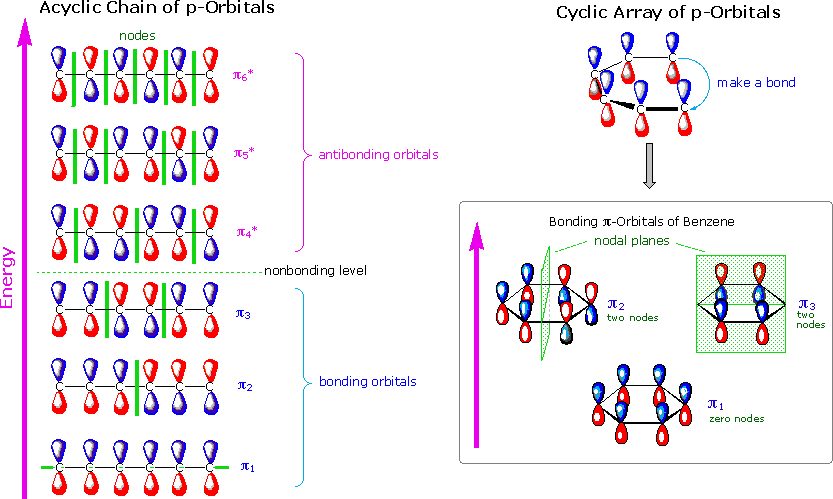
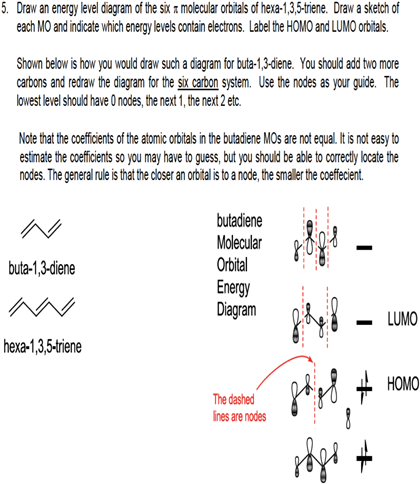
1 3 5 hexatriene pi molecular orbitals. Complete the diagram by adding the appropriate p orbitals in the empty spaces. 6 points write accurate illustrations for each of the following. Show how the molecular orbitals form by drawing the atomic orbitals that combine to form the pi molecular orbitals. In the allyl system ψ 1 is a bonding molecular orbital ψ 2 is a nonbonding molecular orbital and ψ 3 is an antibonding molecular orbital.
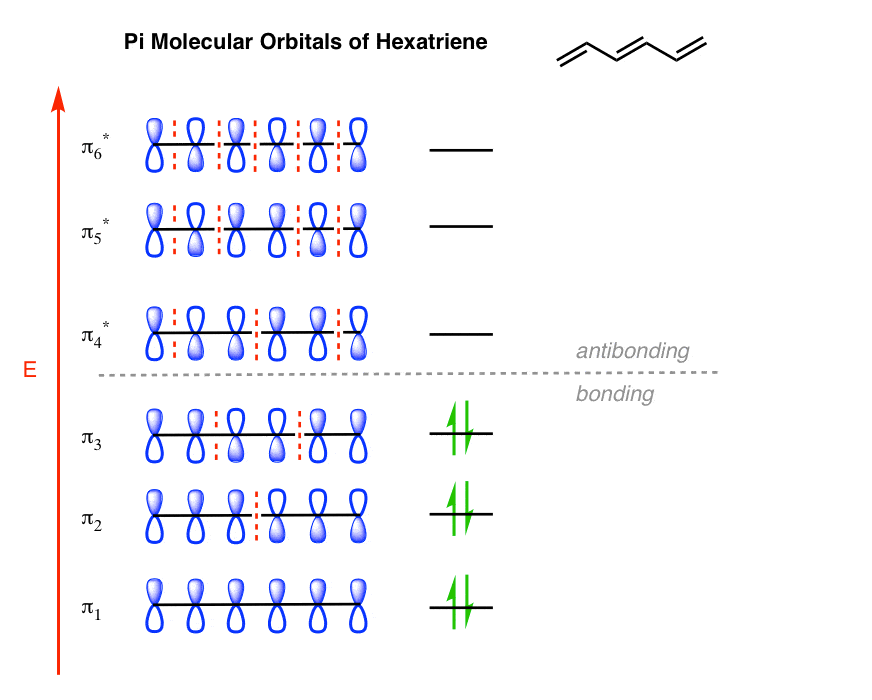
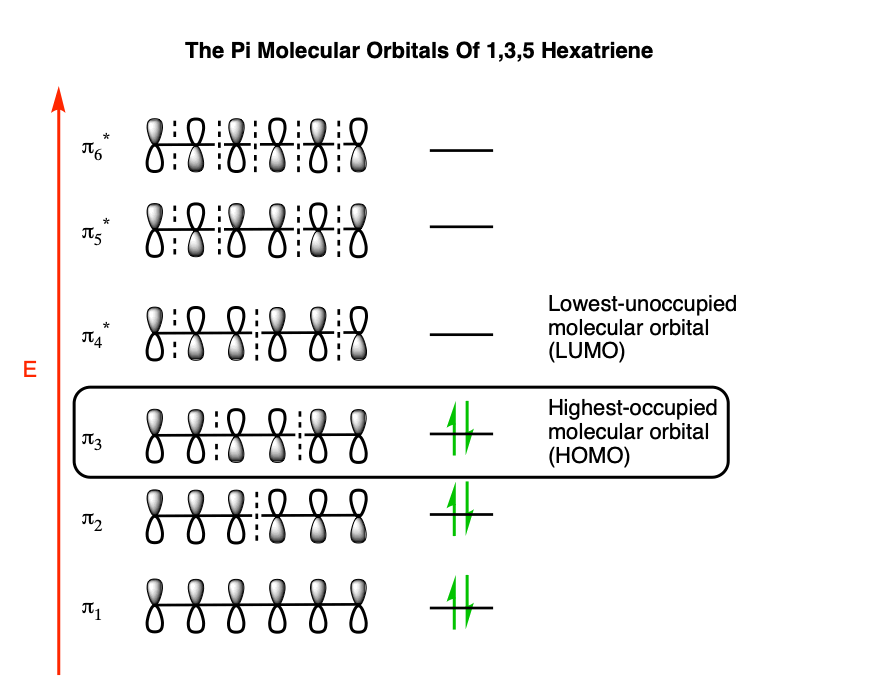
Nonbonding pi molecular orbitals are generally present in conjugated systems with an odd number of atoms. A the energy level diagram for the pi molecular orbitals of 1 3 5 hexatriene. 1 3 butadiene contains two double bonds that are conjugated. The structure of 1 3 5 hexatriene is shown below.
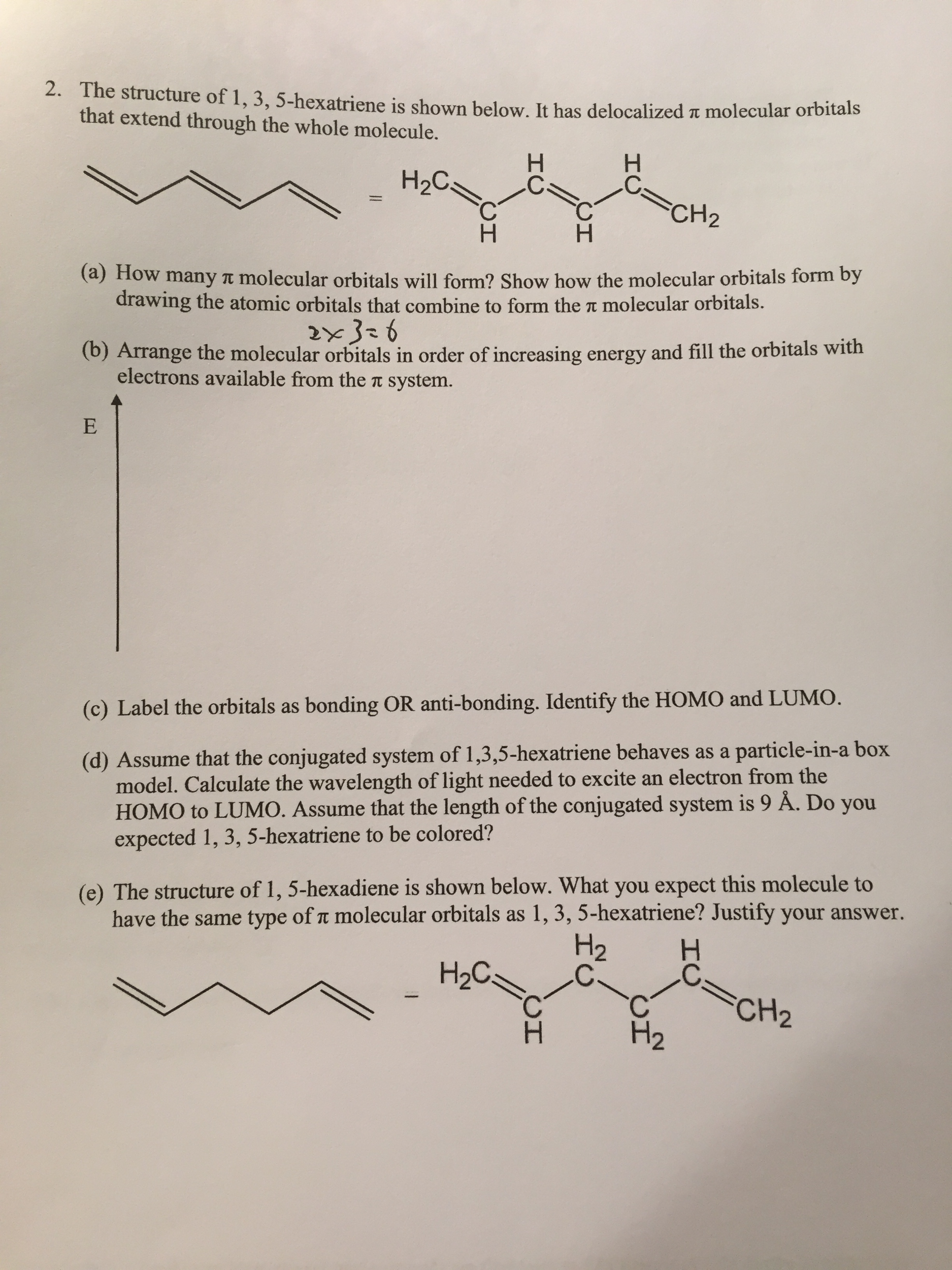
This is one of many videos provided by clutch prep to prepare you to succeed in your college classes. How many pi molecular orbitals will form. The first rule implies that every diagonal. 10 points for 1 3 5 hexatriene.
π molecular orbitals of 1 3 butadiene. Pi molecular orbitals of 1 3 5 hexatriene. Video explaining orbital diagram. They will generally be the median molecular orbital from an energy standpoint and electrons in a nonbonding molecular orbital can be thought of.
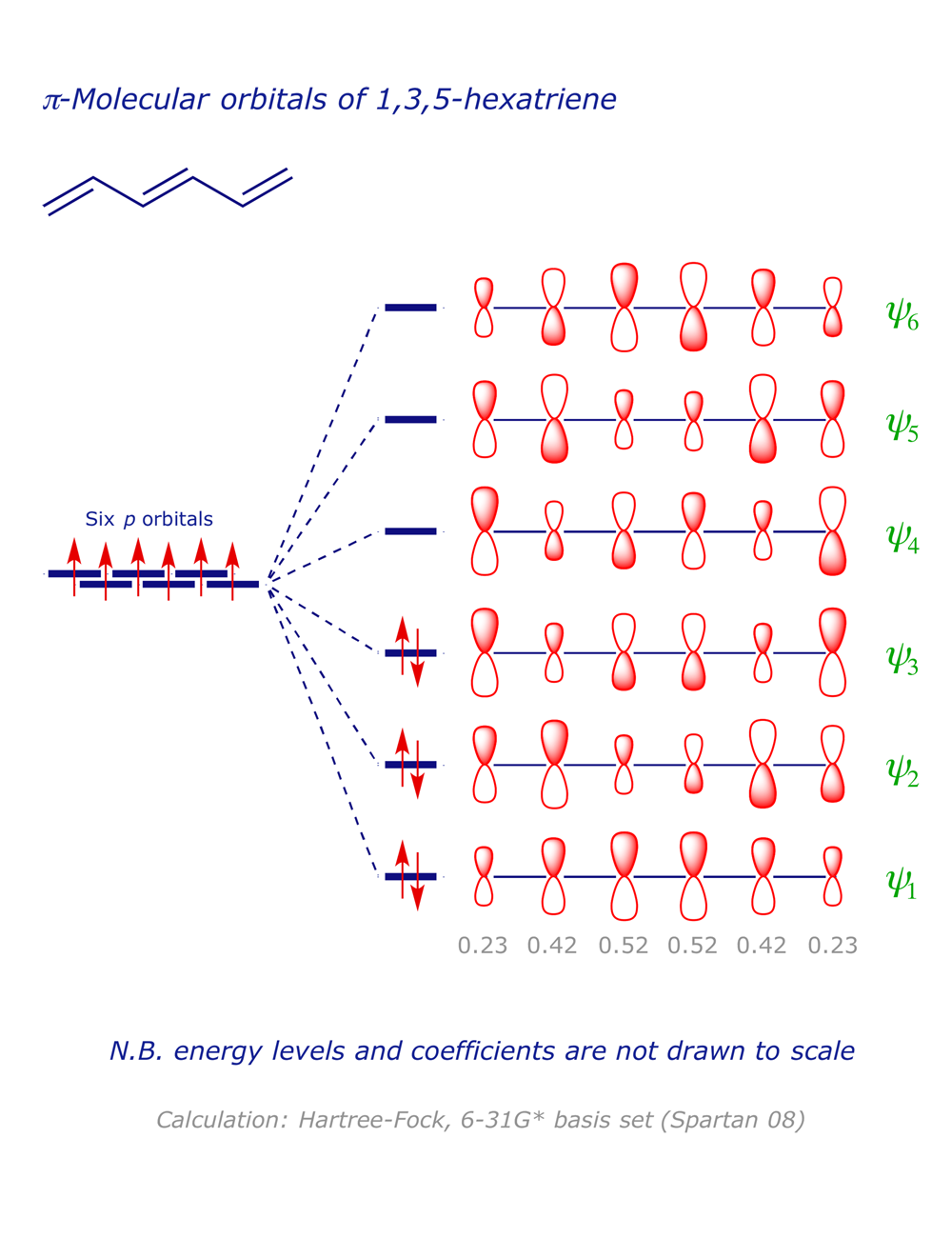
It is a 6 by 6 matrix. With a single sigma bond separating the pi bonds of 1 3 5 hexatriene it is a conjugated system and some of the pi electron density will be delocalized between each of the c c bonds not just those written as double bonds in the lewis structure. 1 2 5 3 6 4 1 each of the mos is a linear combination of 6 pz orbitals cµ 1 µ c2 6 cµ ψµ cµ p z i cµ 3 i µ i 1 c4 cµ 5 µ c6 2 it is relatively easy to work out the hamiltonian. Be certain the symmetry of the diagram is maintained and the orientation of the p orbitals is correct.
6 atoms 1 3 5 hexatriene for organic chemistry. Draw the pi molecular orbital diagram for the compound 1 3 5 hexatriene and identify the homo and the lumo for the compound. An alternative way to consider building the π molecular orbitals is by combining the π molecular orbitals of two ethene molecules. It is built from 4 sp 2 hybridsed c atoms each contributing a p atomic orbital containing 1 electron.