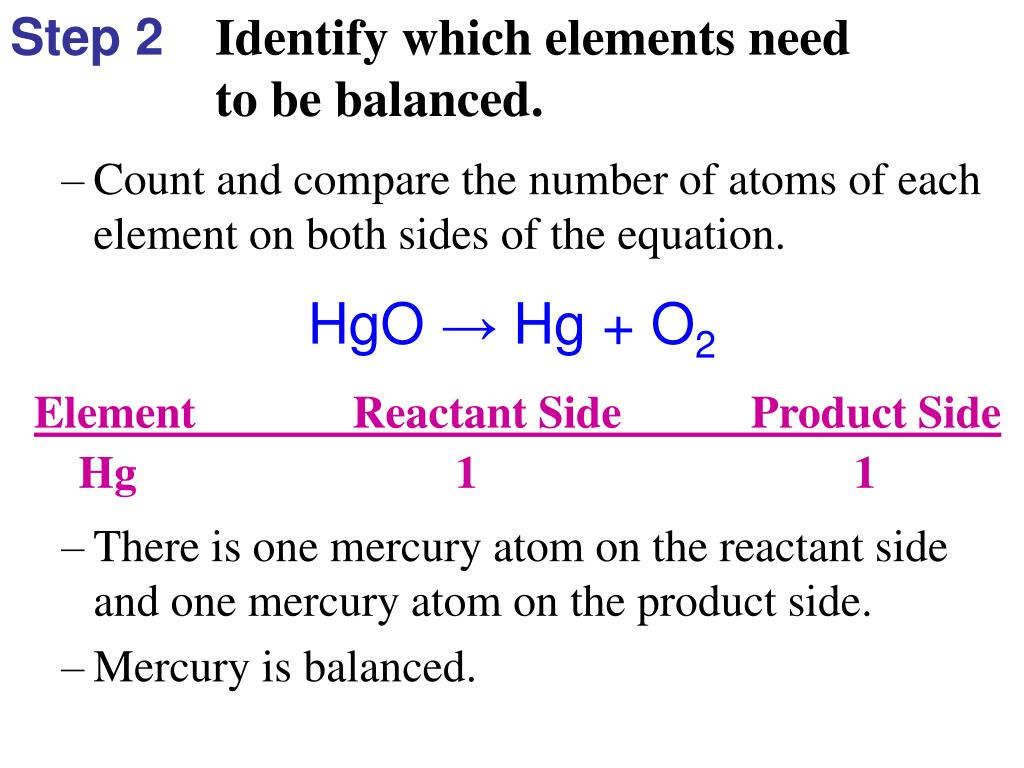
Balance Hgo Hg O2
Enter an equation of a chemical reaction and click balance.
Balance hgo hg o2. Only change the coefficients these are the numbers in front substances. The answer will appear below. The balanced equation will appear above. Fe au co br c o n f.
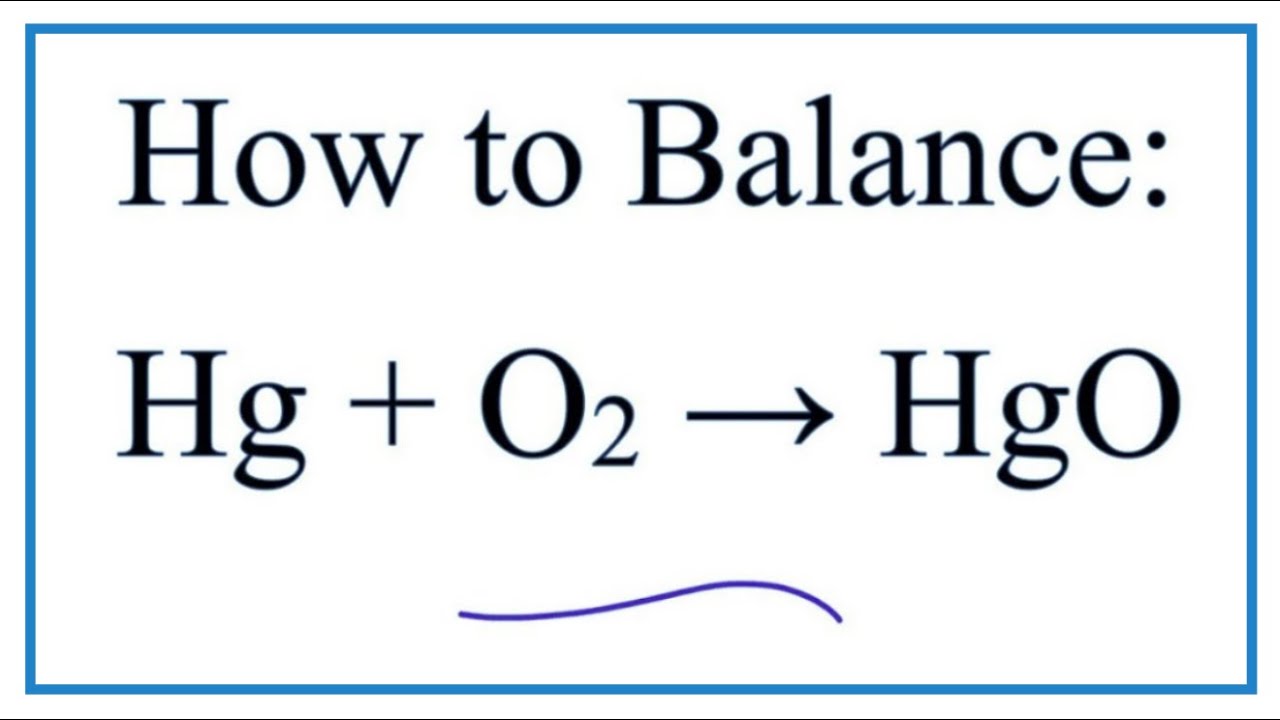
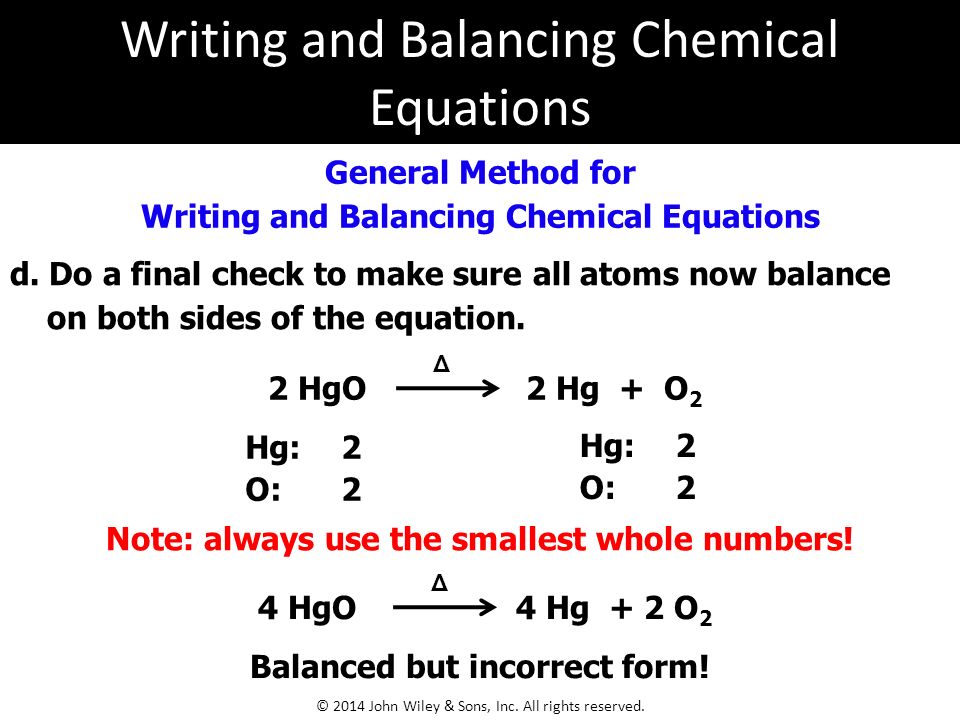
In this video we will balance the equation hg o2 hgo and provide the correct coefficients for each compound. 2hgo hg o2. Always use the upper case for the first character in the element name and the lower case for the second character. We get the balanced 2hgo 2hg o2.
First balance the oxygen so put a 2 in front of the hg on the left side. Balance hgo hg o 2 the law of conservation of mass states that matter cannot be created nor destroyed. To enter an electron into a chemical equation use or e. Ionic charges are not yet supported and will be ignored.
The balanced equation will appear above. Hg o2 8024491 what do you observe when acetic acid is added to neutral fecl3 solution give long answer write nernst equation for the following cell reactions sn 0 050m h 10 020m hyflatm p. To balance a chemical equation enter an equation of a chemical reaction and press the balance button. Multiply the left side by 2 to get 2 oxygen.
Fe au co br c o n f. How to balance hg o2 hgo mercury oxygen gas when balancing chemical equations our goal is to have the same number of each type of atom on both sides of the equation. Ionic charges are not yet supported and will be ignored. Then multiply hg on the right by 2.
Never change the subscripts the small numbers after elements. Co cobalt and co carbon monoxide. Then that unbalances the hg. Fe au co br c o n f.
Hgo hg o2. Balance hgo hg o2. To balance hg o2 hgo you will need to be. Use uppercase for the first character in the element and lowercase for the second character.
In order to balance the chemical equation hgo hg o2 watch this video till end and you ll surely understand how to balance it. At stp for 11 2 litres of a gas pv is equal toa 1rtb 0 75rtс 0 5rtd 0 25rt the cost of the tata salt nacl is rs 20 per kg. There are no more or less atoms at the end of a chemical reaction than there were at the beginning.