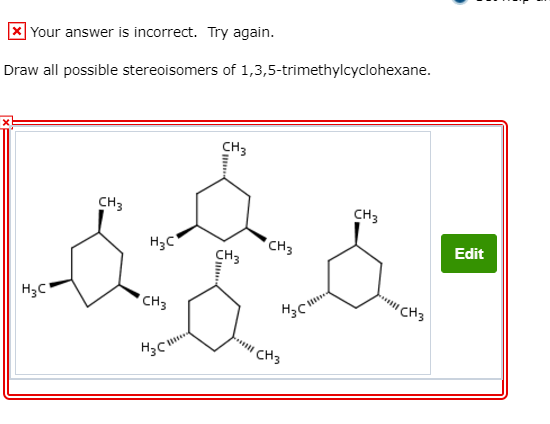
1 3 5 Trimethylcyclohexane Stereoisomers
Become a member and unlock all study answers.
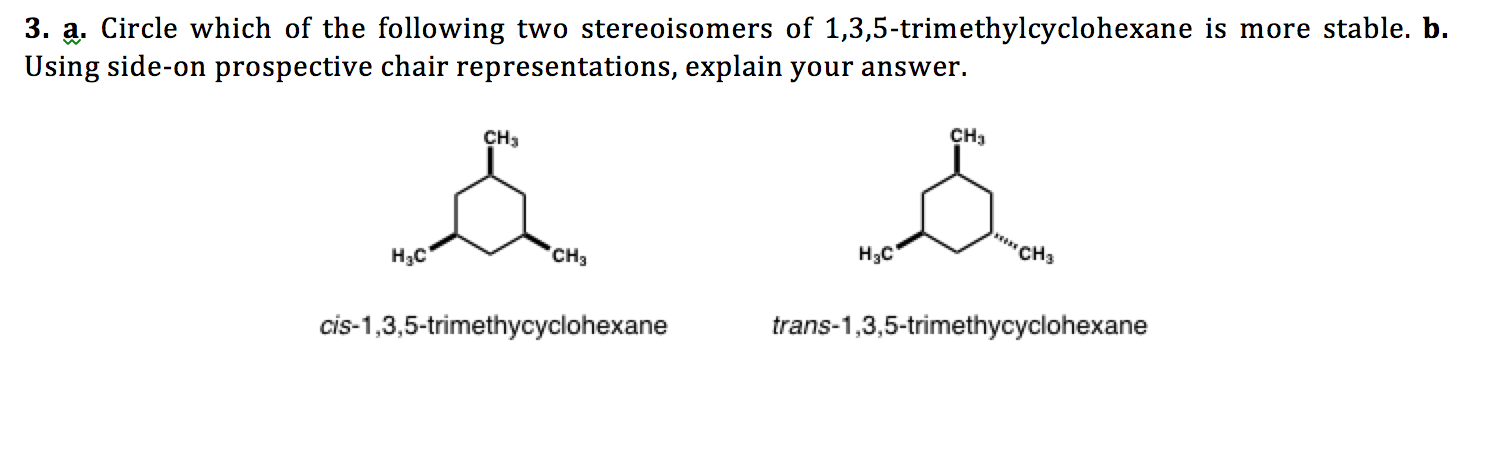
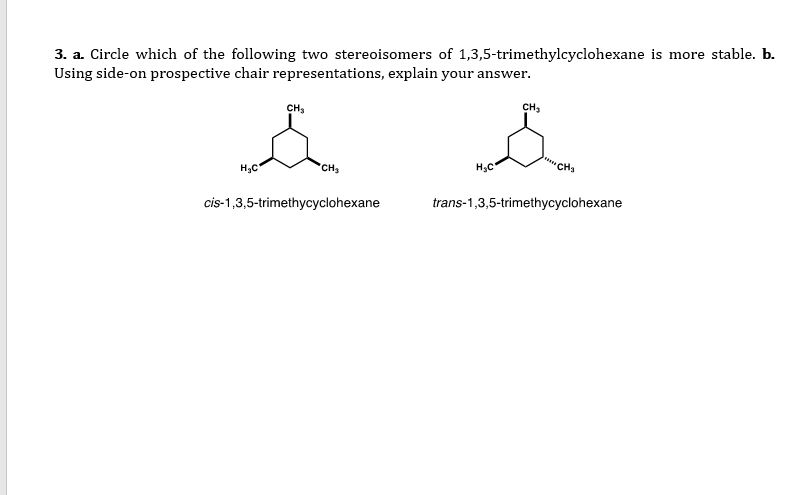
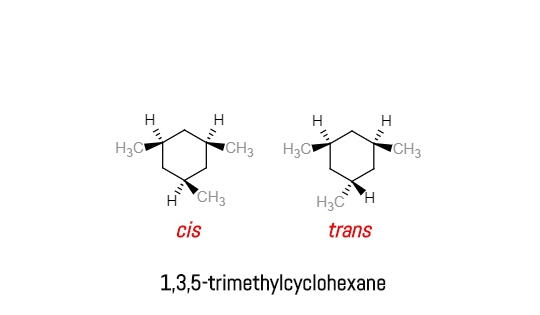
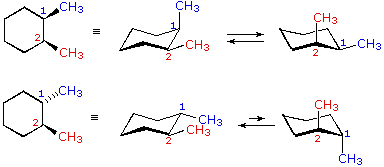
1 3 5 trimethylcyclohexane stereoisomers. In the structure to the left this would be possible with the structure on the right not. If the substance is covered by more than one clh entry e g. δ r h 196 1 0 84. The molecule as a whole isn t really chiral either since its mirror image is superposable onto the original.
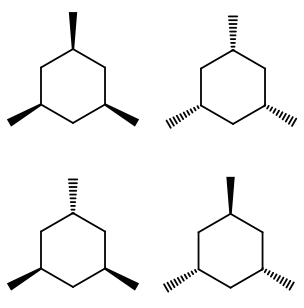
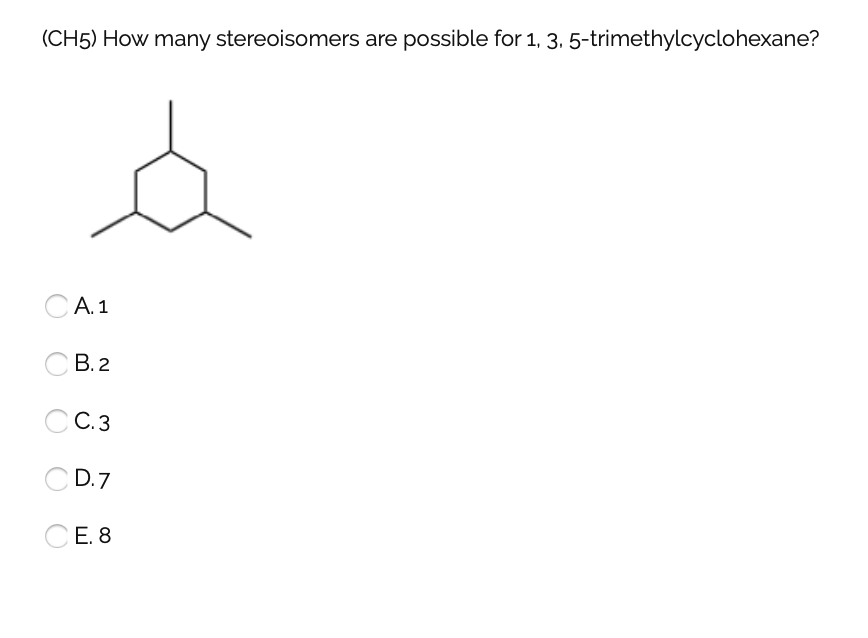
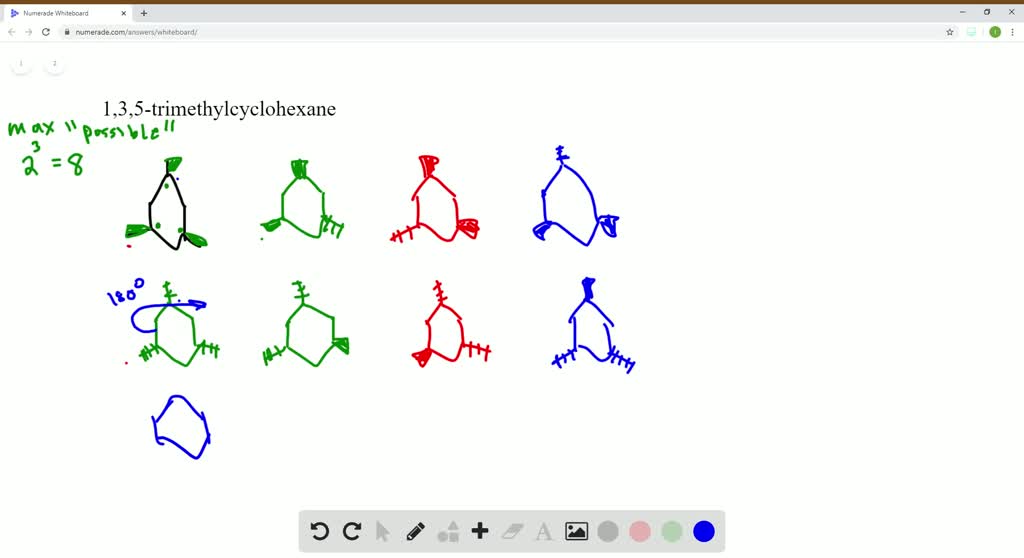
Organic and biological chemistry treatment of the following stereoisomer of 1 bromo 1 2 diphenylpropane with sodium ethoxide in ethanol gives a. At least one methyl group has to be in axial position. 005 011 01 1 and 005 011 02 9 clh information cannot be displayed in the infocard as the difference between the clh classifications requires manual interpretation or verification. To get the number of stereoisomers you just have to use the formula 2 n where n is the number pf chiral carbons.
Gas liquid critical temperatures and a comparison with the theory of rowlinson and sutton j. However you can still align each methyl in the rear or the front and that does shift the chair flip. 1 3 5 trimethylcyclohexane c9h18 cid 35364 structure chemical names physical and chemical properties classification patents literature biological. Try it risk free for 30 days try it risk free ask a question.
1970 2 105. Reanalyzed by cox and pilcher 1970. 1 5e6 oh cm3 half. 2 56e 007 octanol air koa model.
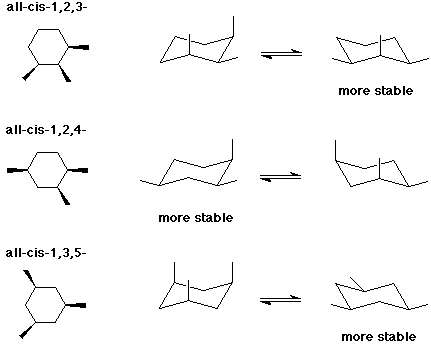
The stereoisomers of 1 3 5 trimethylcyclohexane are. 2 7e 010 fraction sorbed to airborne particulates phi. Well there aren t really any chiral carbons here because each supposed chiral carbon only has three unique substituents methyl hydride methylene not four unique ones. Dolliver gresham et al 1937.
Quantity value units method reference comment. Disodium tetraborate ec no. 215 540 4 is covered by three harmonisations colon. The thermodynamic properties of fluorocarbon hycrocarbon mixtures 3.
3 2e 009 octanol air koa model. Cyclohexane rings tend to be in a chair confirmation with the more bulky groups in equatorial position. 1 15e 007 mackay model. 2 16e 008 atmospheric oxidation 25 deg c aopwin v1 92.
See full answer below.