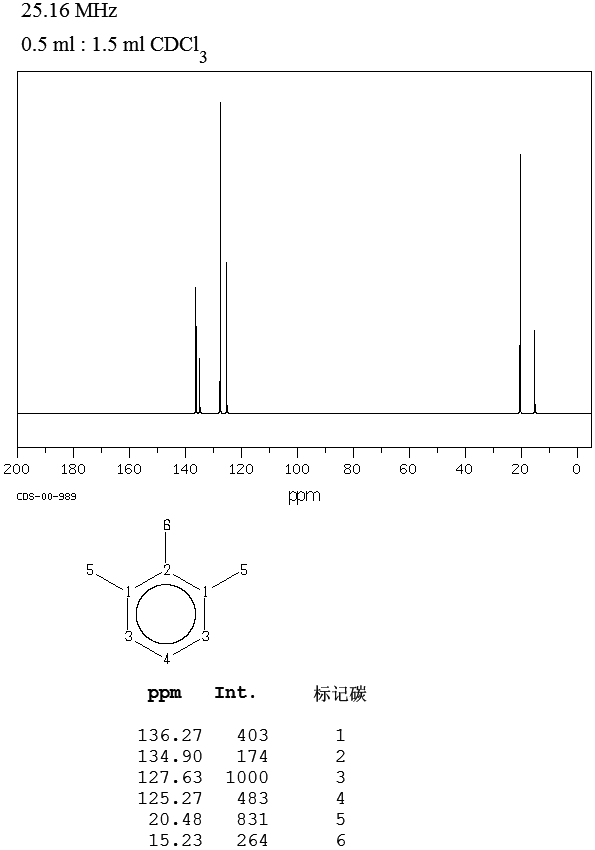
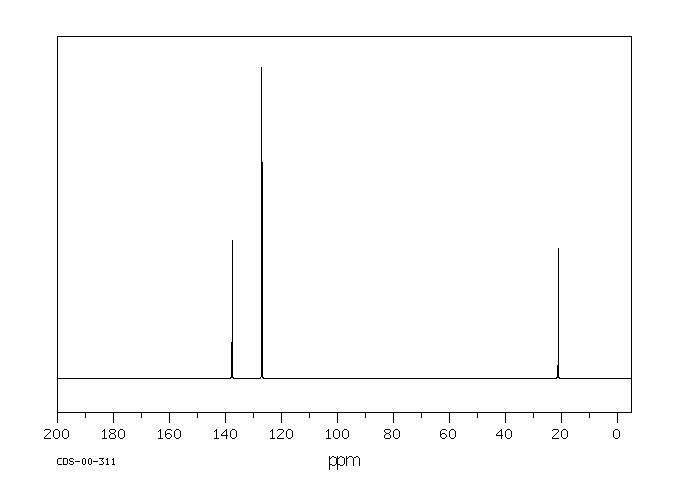
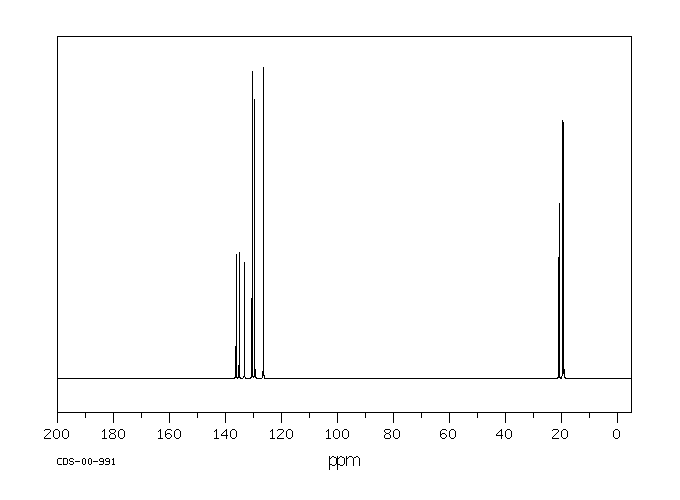
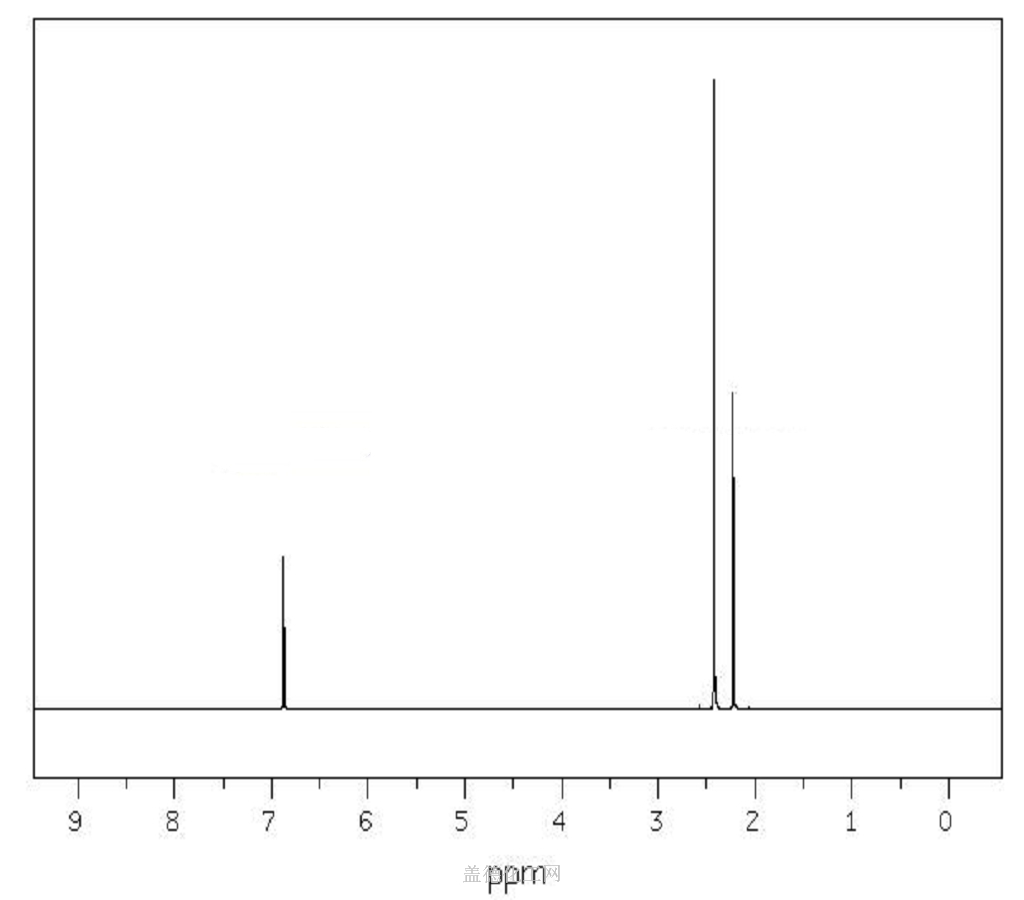
1 3 5 Trimethylbenzene H Nmr

C 18 h 24 cl 4 ru 2 molecular weight.
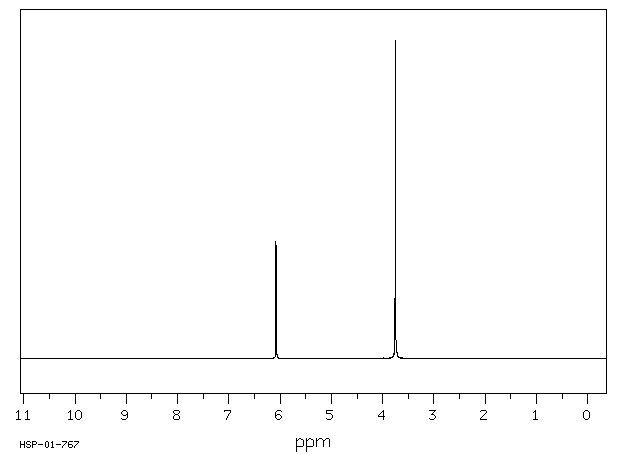
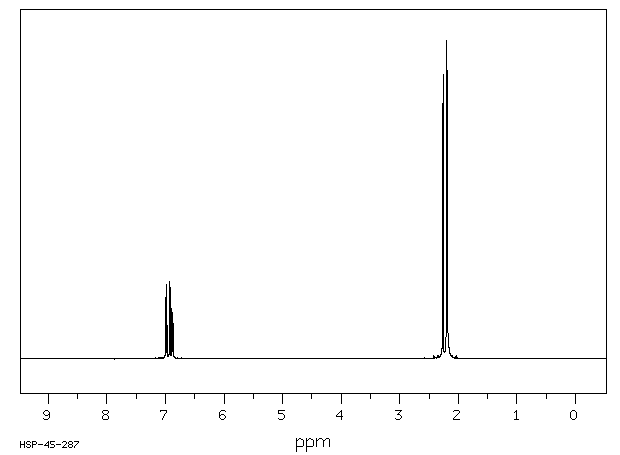
1 3 5 trimethylbenzene h nmr. Niosh rel twa 25 ppm 125 mg m 3 osha pel none. For poly ba a and poly ph a m xylene and 1 3 5 trimethylbenzene compounds have the highest intensities. There will be two peaks peak a at 6 78 peak b at 2 26 peak a is because of aromatic hydrogens and peak b is because of methyl hydrogens. 1 ppm 4 92 mg m 3.
Di μ chlorodichlorobis 1 2 3 4 5 6 η 1 3 5 trimethylbenzene diruthenium ruthenium ii chloride mesitylene dimer empirical formula hill notation. Computed by cactvs 3 4 6 11 pubchem release 2019 06 18 rotatable bond count. In contrast 20 37 of the absorbed dose of 1 2 4 tmb 1 2 3 tmb or 1 3 5 tmb was eliminated via exhalation during controlled inhalation exposures in humans. Hope you basically know the reasons i guess aromatic hydrogens are shifted more 6 78 because of the dielec.
In the case of poly ba a m xylene and 1 3 5 trimethylbenzene are the degradation products that can result directly from the degradation of the polymers as poly ba a has a 2 4 6 tri substituted. In humans tmb metabolites are excreted via urine and respiration. Computed by pubchem 2 1 pubchem release 2019 06 18 monoisotopic mass. Dot id guide.
1 3 5 trimethylbenzene proton full spectrum. Computed by cactvs 3 4 6 11 pubchem release 2019 06 18 hydrogen bond acceptor count.