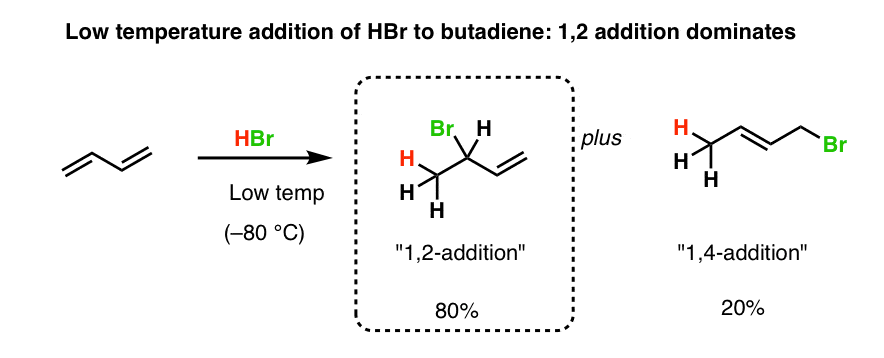
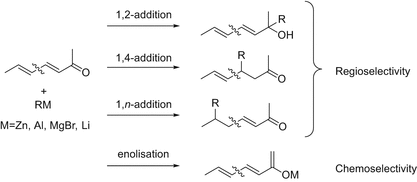
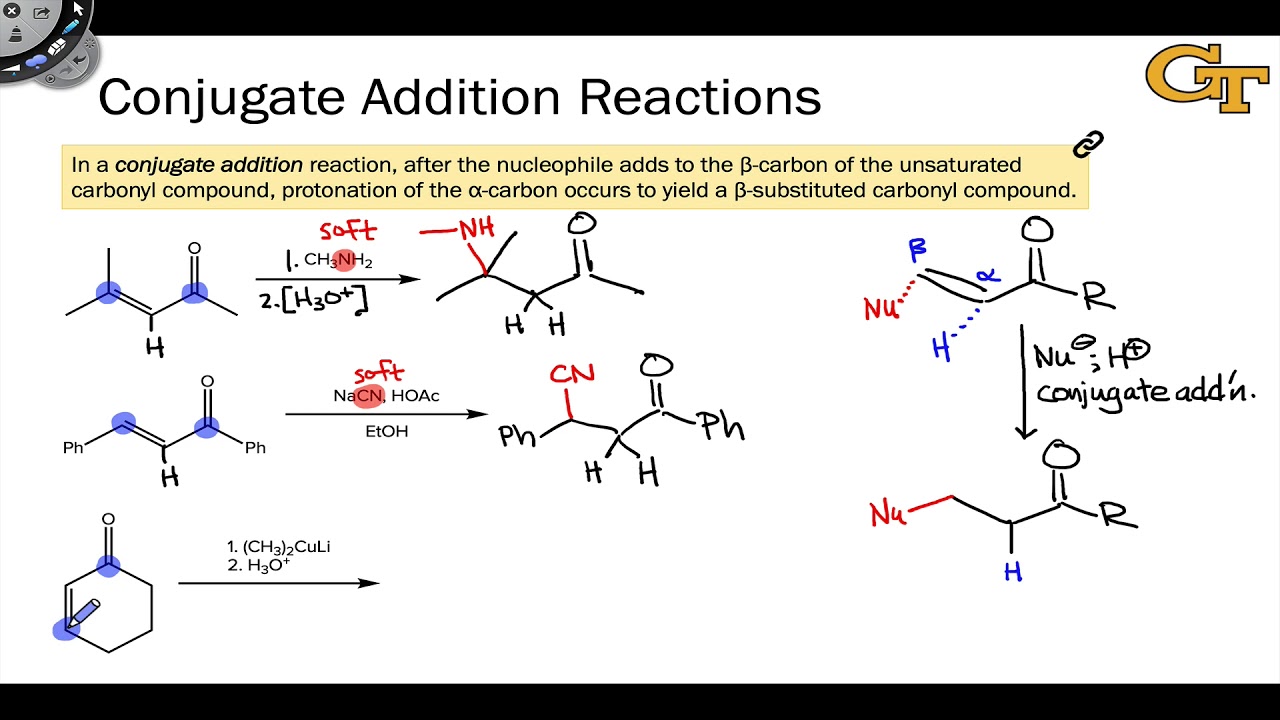
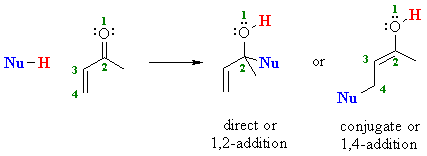
1 2 Vs 1 4 Addition Carbonyl
In this account we summarize our recent studies on cobi catalyzed asymmetric nucleophilic carbonyl addition and tandem reactions.
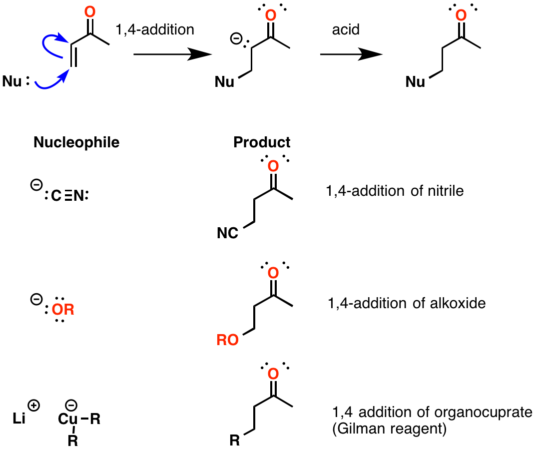
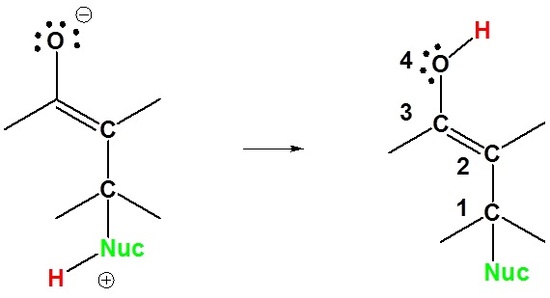
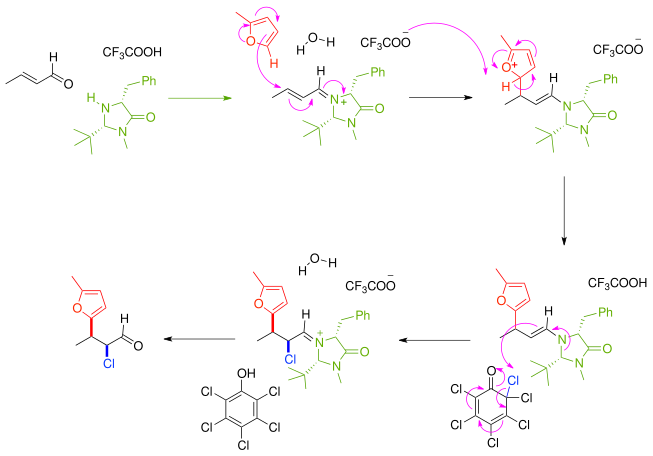
1 2 vs 1 4 addition carbonyl. The power of cobi in catalyzing carbonyl 1 2 or 1 4 addition reactions triggered our interest in developing asymmetric synthetic methodologies to generate versatile enantiomerically enriched compounds. Recent progress in copper i catalysed addition of grignard reagents is reviewed throughout this chapter comparing and contrasting the well established 1 4 selectivity of cu i ligand complexes with the newly. 1 2 and 1 4 additions to carbonyls some of the earliest attempts to understand stereoselectivity in organic reactions were the rationalizations and predictive models made in the early 1950s by curtin 1 cram 2 and prelog 3 to explain the addition of achiral nucleophiles such as grignard reagents to the diastereotopic faces of ketones and aldehydes having a proximal stereocenter. Stability of resulting product.
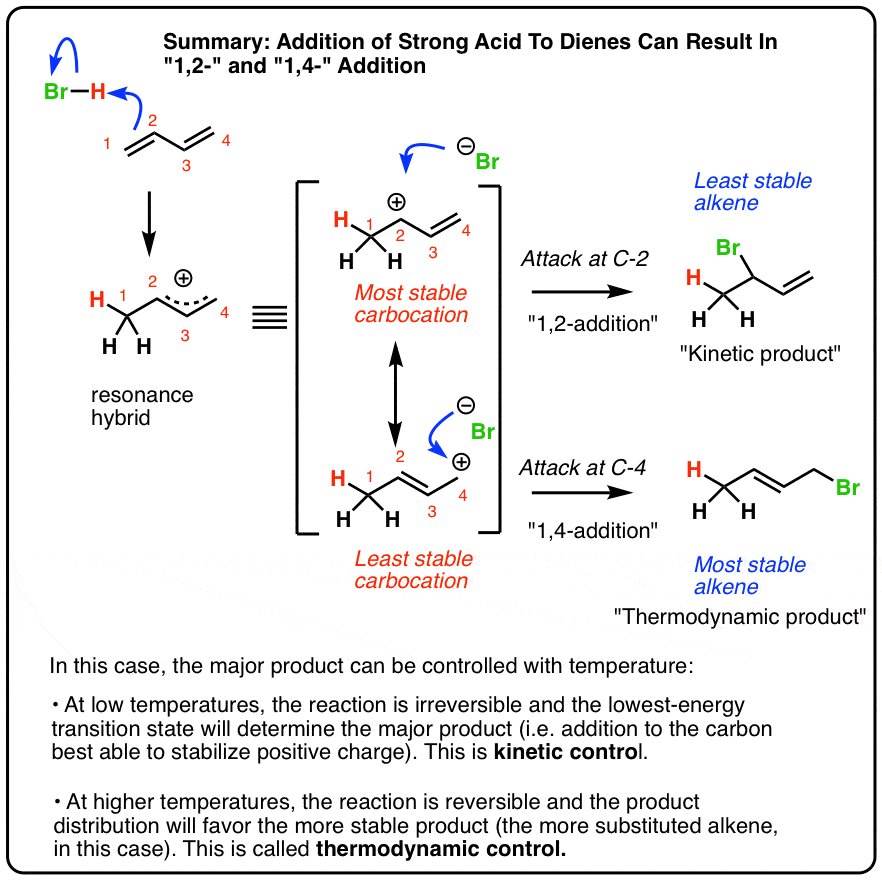
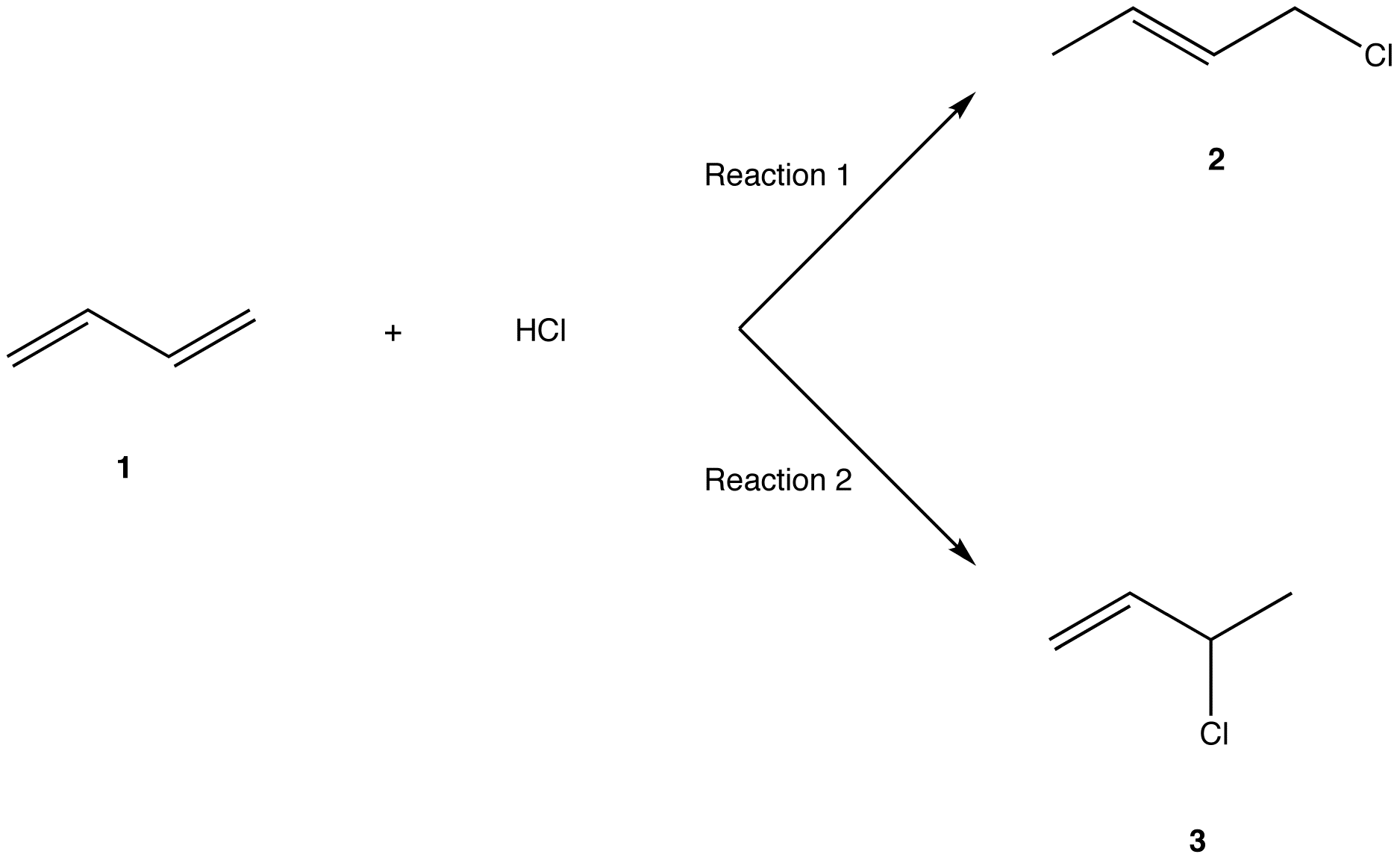
Nucleophilic conjugate addition is a type of organic reaction ordinary nucleophilic additions or 1 2 nucleophilic additions deal mostly with additions to carbonyl compounds. 1 2 and 1 4 addition in the reactions of carbonyl compounds with 1 3 butadiene induced by cerium iv ammonium nitrate. The recent discovery that copper i is able to catalyse the asymmetric 1 2 addition of grignard reagents to α β unsaturated as well as aromatic ketones was a true revelation. This means the competition between 1 2 and 1 4 addition is under thermodynamic control.
In the aldol cross aldol reaction of α β unsaturated carbonyl compound under what circumstances does a 1 2 addition i e. If the nucleophile is a weak base such as alcohols or amines then the 1 2 addition is usually reversible. Due to the influence of the oxygen atom the carbonyl carbon is positively polarized. Since 1 2 additions to the carbonyl group are fast we would expect to find a predominance of 1 2 products from these reactions.
Simple alkene compounds do not show 1 2 reactivity due to lack of polarity unless the alkene is activated with special substituents with α β unsaturated carbonyl compounds such as cyclohexenone it can be deduced from. 1 2 kinetic faster larger partial charge at carbonyl carbon 1 4 thermo slower smaller partial charge at 4 c but ultimately more stable product how determine whether 1 2 or 1 4 addition occurs to ald ketone. Michael condensation carbanion formed attacking β carbon. Thus 1 2 or 1 4 addition occurs in α β unsaturated carbonyl compounds.
These are the following points i considered.